The Integration of Adipocytes into 3D Meat
Introduction:
Texture is one component of consumer acceptance that has lagged compared to traditional products, and is rated one of the most important aspects of consumer acceptance for meat. Scaffolds are essential for the shape of cultured meat and plant-based products; they are the framework. To create a successful meat analogue, scaffolds are essential to creating the stiff, structured feel of a traditional piece of meat. They are what provide the “bite” like a traditional cut. The taste and texture of cultured meat should remain the springy, thick, and satisfying experience that traditional meat is – think of what makes a slice of bacon give a pull, what makes a piece of steak just the right amount of chewy and soft, or what makes a filet of salmon segment itself into delicious, firm pieces. That’s what scaffolding will help cultured meat achieve; without it, there’s no crunch, pull, or chew that makes traditional meat or fish the phenomenon that it is, and consequently there is less consumer acceptance around some substitutes that we have today. As the CTO of Matrix Meats (who develop customizable scaffolds) Jed Johnson says, we don’t want products that are mush.
Cultured, cell-based, or ‘clean’ meat defines a new era of protein production entailing the growth and expansion of animal cells under laboratory conditions. New innovations in cultivated meat technology are rapidly developing to address the challenges facing this burgeoning industry; namely – cost, efficiency, and scalability. The demand for clean meat, fueled by the potential to reduce environmental burdens of meat production, is driving expansive RD efforts. However, many of the products nearing the market lack the texture of whole cuts of meat. The difficulty in growing a steak ex vivo, or outside a living organism, derives from a lack of structured support during culture, which is required to integrate cells in a more physiologically relevant manner. To achieve this, scaffolds are expected to be a necessary component of any cultured meat system.
According to a 2021 study, which also attracted media attention, 80 percent of UK and US consumers surveyed said they would be willing to try cultured meat. 85 percent who were 39 or younger had positive responses, and 89 percent of Gen Z reported they would try it (to which daughter of Willem Van Eelen, "father" of cultured meat, Ira Van Eelen, responded, "why wouldn’t they?") And it’s true - Gen Z is open minded and set on ethical consumption, and they are the next market. On the whole, those surveyed estimated it could account for 40 percent of their diet. In order for these numbers to stabilize or to grow in the future, the industry needs to prove to its consumers that cultured meat products will not only be climate positive and healthy, but will taste, smell, feel, and look appetizing, like the real meat that it is.
However, because the cultured meat industry has not scaled up yet, there are materials that are more or less promising when it comes to creating viable scaffolds for successful products that investors in the field should be aware of. Although there are a huge number of potential scaffold materials and synthesis techniques being proposed, key factors to keep in mind when it comes to cultured meat production are: (1) base material cost, (2) synthesis method cost, (3) synthesis method time, (4) suitability to produce a good final product.
Scaffolds can be made out of mycelium (a mushroom derivative), chitosan (a naturally derived sugar from crustaceans), decellularized plants like spinach leaves (when the framework of the leaf is extracted to be used for a scaffold), soy, and fermented collagen or gelatin. They can be made through mechanical processes such as electrojet spinning, or physical processes such as freeze-drying. In vivo, or within a living organism, cells form higher structures through the connectivity of the extracellular matrix (ECM). The ECM is composed of fibrous proteins that form a hydrogel, or a natural scaffold [1]. Beyond imparting scaffolding, the ECM interacts with cells to give them cues to turn into different, specialized cell types. As such, scaffolding for cultured meat must mimic these messaging factors. However, many challenges arise in choosing a low cost, animal-free material with the physical and biochemical properties that native scaffolding proteins exhibit.
In recent years, several top contenders have emerged attempting to address the scaffolding challenge. These include mushroom derivatives such as mycelium, chitosan, resident natural scaffolding components such as fermented collagen and gelatin, and low-cost plant-based materials like textured soy protein isolate and decellularized plants, which are plants whose ECM has been isolated from other cells to use the frame as a scaffold. Many of these materials require processing into a scaffold-like structure containing pores, aligned fibers, or vasculature. The most common techniques include nanofiber spinning (electrospinning and rotary jet spinning), lyophilized sponges, bioprinting, and decellularizing plant material. Each of these materials and processing methods maintain unique properties and advantages, however some may prove to be too expensive or time consuming to realistically transition to large scale production. This article will report on the application of these materials and methods to the production of cultured meat structures, consider the suitability for industrial adoption, and propose a number of outstanding questions to be addressed in the coming years.
Scaffold Requirements & Challenges
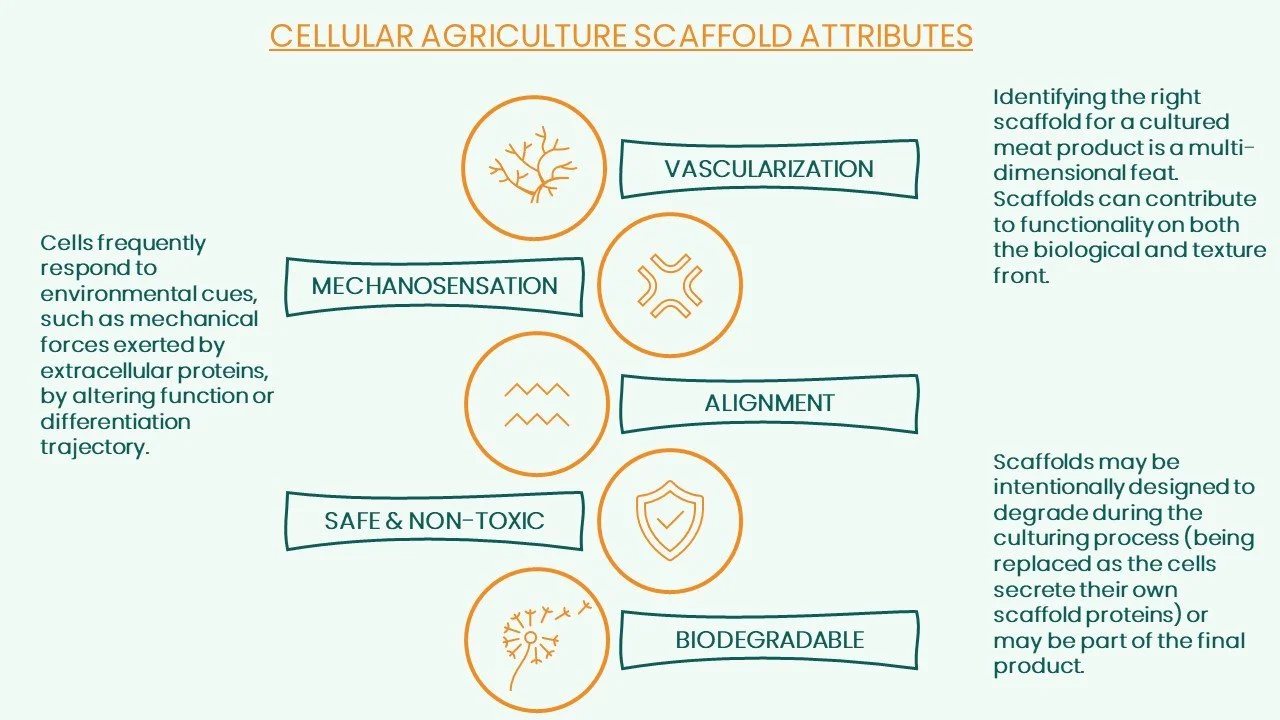
A number of hurdles arise when attempting to deliver a structured meat product, even at an average size. Vascularization is the first major limitation on the horizon, but the challenge has largely been avoided by most companies at the current stage of development through the use of small prototypes. However, as we move towards generating thicker iterations of cultured meat, nutrient diffusion will become limited. While increasing porosity, or sponginess, of the scaffold can aid in the flow of nutrients, if defined transport networks are not included in the structure and nutrients are not being circulated, it could lead to tissue necrosis (cell death) at the center of the product. Yet another major complication of higher order tissue structures is the requirement for multiple cell types. A typical cut of meat will contain several types of cells – muscle cells intercalated with fat cells (marbling) form the bulk, microvasculature, and fibroblasts (the cells that create the framework in animal tissue, make up the connective tissue component). When this process takes place inside a cell of a living organism, these different cells display distinct codes for the specialized function of different ECM proteins [1]. This poses the possibility of designing scaffolds [2] with various cellular niches in mind to promote the growth and differentiation of cells. It would be required to create a functional space for cells to grow that will mimic the native environment of that specific cell type. This may require adding in molecules/proteins to the scaffold that specifically mimic the ECM.
Depending on how this is executed it may increase post-processing time after scaffold assemblyand, based on current prices of fermented scaffold proteins, increase cost. Companies will need to determine if the advantages that functionalization delivers outweigh the associated costs. Next, the structure and organization of the scaffold is itself essential, specifically in ensuring that stem cells turn into muscle. This is reliant upon proper cell alignment, and is a vital component of delivering the appropriate texture of 3D cultured meat. These kinds of scaffolds for the proper construction of muscle cells have already been implemented in the field of tissue engineering; it is not something that is far off what the medical field has already been doing for years. By creating aligned nanofiber scaffolds [3] or microstructured chips containing linear grooves [2], termed topographic activation, muscle constructs similar to native tissue can be obtained. Thus, it is likely that scaffolds for cultured meat will need to be created with cell alignment in mind.
Lastly, the ability of the scaffold to degrade or seamlessly incorporate into the final product is another important element of scaffold design. Two possibilities exist here:
1. Construct a scaffold that maintains its integrity throughout the process of cell growth and differentiation but degrades by the time the product reaches shelves or during the cooking process
2. Construct a scaffold meant to be eaten that may even add to the texture or nutrition profile of the final product
3. Removing the scaffold once product growth is complete is an option that has also been proposed. However, this methodology is often not discussed in the context of a 3D piece of cultured meat, where enzymatic digestion (the breakdown of food) and centrifugation (separating particles from their solution) cannot be optimally applied
Materials Cost
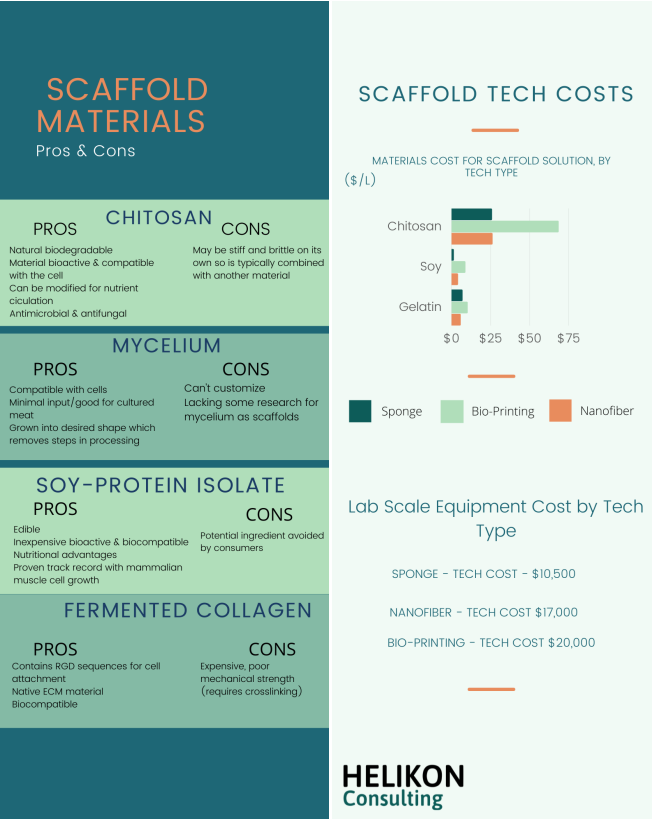
Aside from suitability from a biological context, the cost of materials is important to consider when determining the future of these potential scaffold systems for cultured meat (fig. 2). Cheapest to most expensive:
• When purchased in bulk: plant materials like apples and celery (less than $0.01/g) soy protein isolate ($0.06/g)
• Gelatin from cows is slightly more expensive ($0.25/g), but still relatively cheap since it is produced at such large scale already.
• Vegetal and crustacean chitosan are priced around $2/g.
• Mycelium scaffolds from the Excell culture kit cost $12/g, however mushroom spawn for culturing mycelium can be obtained for extremely low-cost (less than $0.01/g).
• Fermented collagen (produced in E. coli or Yeast expression systems) is extremely expensive ranging from hundreds to thousands of dollars per gram.
At the moment, companies making recombinant collagens for science applications mostly sell human and mouse collagen chains and few offer bovine collagen. While collagen derived from animal tissue is less expensive (bovine: $24/g) and can be more appropriate for early RD phases, companies will eventually need to tackle the issues of costly recombinant protein production or identify a novel source of animal-free collagen.
Evidently, although collagen and other ECM proteins may be the best suited to replicate the natural cell environment, at current prices they are not reasonable scaffold materials. While gelatin is a relatively cheap substitute, it is derived from animal origins. Non-animal derived gelatin is being developed by companies such as Geltor, however it is likely much more expensive than animal-derived gelatin. Plant sources of scaffold material such as apples, soy, and fungi appear to be the most affordable; however, as will be discussed later on, in some instances the cost of scaffold synthesis or processing time affects the appropriateness of these materials for industrial adoption.
Most methods including sponge-like scaffolds, nanofiber spinning, and bio-printing require an initial scaffold solution containing the scaffold material, crosslinking agents, stabilizers, and solubilizers. These additives differ based on the method, so cost differences within a method are predominantly driven by bulk scaffold material cost, while cost differences between methods may be driven by both material cost and additives. Additionally, the cost of initial pilot-scale equipment for each method can differ. Lab-scale lyophilizers for sponge-like scaffolds are more affordable (HarvestRight: $10,500) and less complex machinery, while the minimum cost for labscale equipment for electrospun nanofiber scaffolds ($17,000 [41]) and bio-printed scaffolds (Dr. INVIVO by ROKIT Healthcare: $20,000) is much greater as they are more complex. Thus the overall costs associated with scaffold synthesis is based on the price of material, additional required reagents which varies by method, and finally the technology cost.
Companies & Patents
3D cultured meat products are being pursued by several companies, although each has developed a unique strategy for scaffolding. Companies like Matrix Meats and Ecovative Design specialize in scaffold fabrication to support the growth of a variety of cell types and are not themselves producing cultured meat products.
• Matrix Meats, which collected a seed round in December 2020, employs electrospinning technology that can be used with many base materials to produce custom nanofiber scaffolds while Ecovative’s Excell mycelium-based scaffold is a research-application-only product at this stage
• Based on a number of patents held by Ecovative ($90.1M in Series D funding) that were published last year (2020) detailing methods of mycelium growth, scaffold formation, and bioreactor design, they are clearly expanding upon their current technology and processes [27]
• Boston Meats, which has raised two rounds, the last of which was a pre-seed round in Nov 2020, grew out of a tissue engineering lab at Harvard University appears to be testing gelatin nanofiber scaffolds created from rotary jet spinning.10 It is unclear whether they intend to grow cultured meat or focus on scaffold technology
Some companies are tackling all aspects of production in house, including scaffolding, growth media, and cell lines, to produce a 3D meat product.
• Aleph Farms, which has a total of $14.4M in Series A funding, previously utilized soy protein isolate scaffolds to grow a mix of muscle and endothelial cells13 and has recently unveiledtheir first 3D bioprinted steak28. Based on previous press releases, they appeared to be testing other plant-based scaffolds29 but may now be using an undisclosed bio ink containing their cell types. They currently hold patents describing the combination of multiple cell types on a 3D porous scaffold [30], outlining a method for the mass production of pluripotent stem cells from non-human origins31, and for culture media aimed at improving the nutritional value of cultured meat [32]
• Similarly, BlueNalu, which has a total of $84.8M, is developing finfish cell lines and 3D cultured products. Their technology includes 3D bioprinting and cold-extrusion, but the composition of the bio ink is unclear33. While no patents are published as of yet, they are likely in the process of filing. Another applicable area of scaffolding for meat products is in plant-based and hybrid meat technology
• Meati Foods, which has $59.1M in Series A funding, are creating plant-based steaks using mycelium scaffolds
• SuperMeat, which has $4.2M in seed funding, holds a patent for hybrid foodstuff combining plant materials with no more than 30% animal cells [34]
For products that are entirely plant-based, scaffolds take on an entirely different purpose where texture and taste become much more important, whereas in hybrid meat the plant substance would still need to act as a cell supportive scaffold.
Scaffold Materials
The ideal scaffold material for cultured meat is a substance that provides alignment and nutrient diffusion, can easily be functionalized, is edible, and inexpensive. The ability of the material to sustain structure and function in large bioreactors for scaled production is also important to consider. In this next section we will discuss the characteristics of the apparent frontrunner scaffold materials and their advantages and/or disadvantages in the application to cultured meat. Scaffolds from Plants
Soy
An inexpensive byproduct of soybean processing, soy protein isolate (SPI) has become more popularas a scaffold choice in recent years. SPI is biocompatible, edible, and promotes cell attachment and multiplication [12]. Furthermore, when processed into sponges by freeze-drying, SPI scaffolds are very porous which aids in cell attachment onto the scaffold and passing nutrients. While the nutritional profile and added protein content of SPI may act as an additional advantage over other scaffold materials, soy products are avoided by some consumers due to allergies or concerns about phytoestrogens.
SPI has been heavily investigated in biomedical and tissue engineering applications due to its ability to support cell growth. SPI can be used with multiple technologies, such as hydrogel and nanofiber scaffolds. For cultured meat purposes, 3D SPI scaffolds have been shown to support co-culture and differentiation of multiple cell types found in cows [13]. This study also demonstrated the important contribution of supporting cell types like fibroblasts to generating a realistic microenvironment. These are important considerations to keep in mind when choosing a scaffold material, especially one that is designed to degrade and be replaced by a natural scaffold that the cells produce.
Fruits & Vegetables
3D scaffolds created from decellularized plant materials such as leaves, apples, and celery have also been gaining traction due to abundance, structural diversity, and low cost. Plants are particularly valuable for the vascular network which ensures nutrient diffusion. Disadvantages of decellularized plant scaffolds include slow break down and limitations on scaffold size and shape within current processing methods. Furthermore, much of the published work involving decellularized plant scaffolds focuses on proof-of-concept feats rather than scaled production potential. Scaffolds from Fungi, Insects, and Crustaceans
Chitosan
A popular material in tissue engineering research, chitosan is a natural sugar derived from chitin, found in crustacean shells, insects, and fungi. Chitosan is often used in medicine and is antimicrobial and antifungal. Some applications include treating high blood pressure and obesity. It is porous and the size of pores can be modified, which is beneficial for passing nutrients. It is not harmful to the cell and is non-toxic and does not cause allergies. By altering certain chemical properties of chitosan, factors like how fast the scaffold will dissolve, how well the cells will stick together, and how well the cells will grow can be fine-tuned [4.]
A wealth of research exists describing the application of chitosan based scaffolds for tissue regeneration purposes, which will propel its swift application in food technologies. Chitosan scaffolds exist in many forms, and it successfully promotes the formation of muscle; however it is important to note that it is typically combined with a secondary substance such as gelatin, or has to undergo a process where the protiens are bound together following synthesis [3,5]. This is due to the stiff nature of chitosan, which exerts mechanical forces much greater than found in a typical cell and could alter cell function and specialization. In addition to tissue regeneration work, recent research has shown the ability of chitosan sponges to support insect muscle cell growth and specialization for cultured meat applications [6].
Mycelium
Another mushroom derived scaffold material is mycelium, which is the vegetative component of fungi. In the past decade, mycelium has become an attractive substance for creating green biomaterials such as packaging and insulation [7]. Its biodegradability and environmentally friendly growth process require minimal input, making it an ideal material for sustainable cultured meat production.
Like chitosan, mycelium portrays bioactive properties and antibacterial and antifungal properties, while maintaining its cytocompatibility, which means it is not harmful to the cell. However, unlike chitosan which is an extract of fungi and must be processed into scaffold structures, mycelium is a living organism that is typically grown to the desired shape. This removes many of the processing steps required, but also diminishes the ability to customize certain qualities. Companies like Ecovative Design, which has raised $90.1M over 12 rounds, are targeting mycelium for a number of applications, including cultured meat. Although stating that their mycelium Excell™ scaffolds kits are compatible with muscle and fat cells and have been tested by several companies and research labs, little to no peer-reviewed data is available. What published works do exist regarding mycelium in cell culture applications do not concern scaffolds [8].
Scaffolds from Animals
Fermented Collagen & Gelatin
Of all scaffolding materials discussed in this report, recombinant collagen, produced in yeast, and gelatin, which is formed by collagen denaturation, most similarly mirror ECM already found in living organisms. A vastly abundant protein found in the ECM, the 29 species of collagen play critical roles in cell growth and specialization, both highly important to a successful product [9]. Because collagen is a naturally derived polymer it is biocompatible, biodegradable, and non-toxic, making it a great option for scaffolding.
Disadvantages of collagen: its high cost to synthesize and poor mechanical strength, causing it to require additional processing steps. Conversely, gelatin is quite affordable when derived from animal sources and maintains many of the advantages of collagen polymers. Unfortunately, very few companies are producing affordable animal-free gelatin at scale. Both recombinant collagen and gelatin have been widely used in scaffolding for tissue regeneration studies in the medical field. Many collagen scaffold synthesis techniques have been established including nanofiber extrusion by spinning, bioprinting, hydrogels, and sponges. Several 3D scaffolds incorporating animal-derived gelatin have been tested for application to cultured meat. Rabbit and bovine muscle cells attach and multiply on edible gelatin nanofiber scaffolds, although specific fiber lengths are needed to cue the cells to align properly [10]. Another study found that mouse muscle cells multiplied well on hydrogel scaffolds containing salmon gelatin and alginate [11]. Neither publication illustrated whether or not cells differentiated on 3D scaffolds, though.
In tissue regeneration applications, several plant materials have been shown to successfully support the growth and specialization of multiple cell types, including fat cells, and stimulate the alignment of muscle cells [14,15]. However, it appears that certain plant materials are better suited to supporting different cell types based on mechanical stiffness, which may influence ability to grow multiple cell types together [16]. Overall, there is a great deal of further research needed to demonstrate the potential of decellularized plant scaffolds for use in cellular agriculture.
Technology and Processing Methods
Many of the described scaffold materials can be used for various synthesis methods to create 3D scaffolds. The most common scaffold synthesis techniques are described below including a discussion of the potential steps following scaffold synthesis that may be required as well as estimated time to produce.
Mycelium Based Foam
Mycelium culture is introduced to a substrate, typically water and nutrients, and allowed to grow for around a week. Then pieces of the growing scaffold are placed into molds of a desired shape and allowed to grow again for several days. Once at the desired size and shape, the mycelium sheets are dried for a few hours to end the growth phase [17]. While this protocol is relatively simple, it can take almost two weeks from inoculation to drying to obtain full scaffolds. Additionally, the growing environment of mycelium must be carefully controlled, including humidity and temperature, which may require special equipment when scaled to industrial production.
Electrospinning Rotary Jet Spinning
Electrospinning (ES) is a nanofiber production method that utilizes high electrostatic charge applied to a polymer solution to extrude nanofibers from a needle [18].
This technique is advantageous:
• It can be applied to many types of solutions
• Creates a scaffold of high surface area to volume ratio
• Can be designed to produce certain orientations and patterns of nanofibers
• Has been extensively employed in regenerative medicine and therefore the technology has rapidly evolved
There are now multiple types of ES machines that can work at higher speeds or with more accuracy in patterning fibers, such as nozzle-free and near-field ES. As this method becomes more utilized in industries new innovations will likely arise to increase output efficiency dramatically.
Rotary jet spinning (RJS) is a new technology that uses mechanical spinning to produce fibers. RJS is as much as 50 times faster than ES, is not quite as limited as is ES, and does not use high voltage electricity [18]. However, ES has been reported to produce much thinner and more consistent fibers than RJS depending on the solution [18]. While ES has been widely used to produce gelatin, collagen, chitosan, soy protein, and fibers and composites, RJS has not been applied as extensively yet. While spinning techniques for scaffold synthesis are on the rise, how fast these machines can realistically function at scale to produce 3D scaffolds is still under question. Additionally, these fiber scaffolds often require post-processing steps which drive up production time.
Decellularization
Decellularization is a process by which cells are removed from a tissue leaving only the ECM framework behind, so that the ECM can be used as a framework. Recently, decellularized plant material has been investigated for cultured meat applications; however this methodology requires further tuning to be able to scale up. The protocol is very simple but also very time consuming. First, plant material is cut to the desired shape, soaked in a solution for several days with agitation to remove cells, washed with water, then finally incubated with calcium chloride for another day to remove residual surfactant before being washed again [14]. While a low-cost technique that uses low-cost materials, there is little information as of yet concerning ways to cut down the processing time, or to scale production up.
Sponges
Sponge-like scaffolds are another popular technique compatible with many of the discussed materials. Sponges are typically synthesized from a solution (chitosan, collagen) or homogenate (soy protein isolate) that is poured into molds and freeze-dried, followed by soaking of the sponge in buffers or water before cell seeding [6]. While this process can become time consuming depending on the number and duration of soaking steps, the freeze-drying component is already used at scale in other areas of the food industry and therefore could be a promising method of scaffold fabrication.
Hydrogels
Hydrogels are networks that retain water and can be formed from many different solutions (chitosan, cellulose, collagen, etc.) combined with physical or chemical gelling. Many properties of hydrogels can be modified, making them suitable scaffolds for cellular agriculture applications [19]. Depending on the solution, these chemical and physical changes can be initiated by temperature, UV light, or addition of chemical crosslinkers. Hydrogels can then be dried or freeze-dried [19].
Bioprinting & Bioink
Bioprinting is heavily employed in tissue engineering applications and has recently been adopted by several companies involved in cultured meat production. This is a method whereby cells and bio ink are simultaneously deposited in a patterned 2D manner and can be printed in layers to achieve a 3D form.
There are three classes of bioprinting machines:
• Laser-assisted
• Inkjet
• Extrusion-based [20]
The main consideration of bioprinting is the composition of the bio ink both for the biological function and the physical properties, such as how thick the ink is. The different types of bioprinters display varying temperatures, pressures, and tendencies to clog, all factors to consider in selecting a bio ink and cell type [20]. Owing to the detailed investigation of bioprinting for tissue regeneration purposes, there is a vast amount of knowledge available for many cell types and bio ink materials already. Furthermore, as this methodology is currently being scaled up for biomedical applications, more industrial technology and production plans will be developed for the cultured meat industry to take advantage of.
Cost & Rate Considerations
When identifying potential scaffold synthesis technologies, companies in the cultured meat space will need to consider compatibility with their material, costs, production rate, and scalability. The most appropriate synthesis process for industrial scale would include affordable materials and reagents, minimal complex or expensive equipment, and fast production timelines. Each of the discussed methods champion certain categories while coming up short in others. For instance, bioprinting and electrospinning employ very complex and expensive machinery, but run continuously so are only limited by their rate. However, it should be noted that nanofiber spinning rates are extremely variable and depend on many parameters like polymer type and concentration, collection method (plate, 3D mold, liquid), applied voltage, and more so it can be very difficult to estimate time to produce a scaffold of a certain material based on available data. In contrast, decellularizing and mycelium foam growth protocols utilize very little specialized equipment but can take up to a full week or more to produce scaffolds and are limited by the capacity of the particular equipment in use.
Thus, comparing rates theoretically can quickly become difficult as techniques can be rate limited or volume limited. While lyophilization, drying, and decellularization processes are generally limited by volume (equipment or tank capacity), ES, RJS, and bioprinting are limited by rate. Within each type, price per capacity or price per rate can be determined based on cost of starting equipment. Lypholization equipment (used in sponge or hydrogel scaffold processing) is much more expensive when scaled up than conventional drying ovens (used in mycelium foam drying) per square meter of available space, which actually becomes cheaper as scale increases. In rate-limited methods, bioprinting appears much more affordable at scale per rate (meters produced per minute) compared to electrospinning based on the pricing information we sourced (table 1).
Remaining Questions & Unknowns
There are many subjects that remain to be addressed through RD efforts to determine the feasibility of some of these proposed materials and processes. As discussed earlier, the benefit of functionalizing a scaffold must be compared to its expense. For different scaffold materials and cell types this balance may change. Certain cells require expensive growth factors to grow. Creating an ECM may reduce the need for growth factors, as ECM functions as a storage and release site for growth factors and protects them from degradation [39]. Such aspects and analyses are important to consider when deciding whether to functionalize or not. Another major question that may be addressed in the coming years is the actual necessity of exogenous scaffolds. Technological advancements may arise allowing for cultured meat at scale without any scaffold material aside from the endogenous ECM secreted from the cells themselves. There are currently published methods which propose such systems, such as -SACS40. Depending on the cost of such protocols, going scaffold-free may remove some of the expensive processes involved in growing 3D cultured meat.
Another unknown at the moment is the potential future cost reduction of some of the more expensive scaffold materials with heavy investment in industrializing production, especially for animal-free origin materials. For instance, chitosan has traditionally been derived from crustacean waste, and only recently vegetal derived chitosan products have been hitting the market in bulk. As demand increases for animal-free polymers and large scale production becomes established, costs may diminish. Conversely, materials like gelatin which are currently inexpensive but derived from animals will require new systems of production entirely if the overall cultured meat product is to be animal-free. As this industry develops we may observe drastic changes in the cost landscape of some of these materials.
Next Steps & Concluding Remarks
As more companies in the space move to create a cultured steak of realistic size, several barriers must be overcome. These include designing scaffolds that allow for nutrient circulation, that support the culture of multiple cell types (muscle, fat, and supporting cells), that are affordable, and ultimately that produce a delicious product. In the near future it will be important to improve the current technology for scaffold synthesis to become more efficient and cost-effective, while also advancing the technology for animal-free sources in order to drive down costs.
Although there are a huge number of potential scaffold materials and synthesis techniques being proposed, key factors to keep in mind when it comes to cultured meat production are: (1) base material cost, (2) synthesis method cost, (3) synthesis method time, (4) suitability to produce a good final product. Each of the materials discussed harbor unique properties making them advantageous for cultured meat applications, whether it be the complex vasculature of decellularized plants, antimicrobial aspects of chitosan and mycelium, or the specialized function of collagen and ECM proteins. Additionally, many of the materials can be applied to a wide variety of synthesis techniques creating a diverse pool of scaffold choices. While patents are beginning to emerge covering some of these approaches, we anticipate many more innovative designs on the horizon.
Bibliography
1 Frantz C, Stewart KM, Weaver VM. The extracellular matrix at a glance. J Cell Sci. Published online 2010. doi:10.1242/jcs.023820
2 Zhao Y, Zeng H, Nam J, Agarwal S. Fabrication of skeletal muscle constructs by topographic activation of cell alignment. Biotechnol Bioeng. Published online 2009. doi:10.1002/bit.22080
3 Cooper A, Jana S, Bhattarai N, Zhang M. Aligned chitosan-based nanofibers for enhanced myogenesis. J Mater Chem. Published online 2010. doi:10.1039/c0jm01841d
4 Rodríguez-Vázquez M, Vega-Ruiz B, Ramos-Zúñiga R, Saldaña-Koppel DA, Quiñones-Olvera LF. Chitosan and Its Potential Use as a Scaffold for Tissue Engineering in Regenerative Medicine. Biomed Res Int. Published online 2015. doi:10.1155/2015/821279
5 Hajiabbas M, Mashayekhan S, Nazaripouya A, et al. Chitosan-gelatin sheets as scaffolds for muscle tissue engineering. Artif Cells, Nanomedicine Biotechnol. Published online 2015. doi:10.3109/21691401.2013.852101
6 Rubio NR, Fish KD, Trimmer BA, Kaplan DL. In Vitro Insect Muscle for Tissue Engineering Applications. ACS Biomater Sci Eng. Published online 2019. doi:10.1021/acsbiomaterials.8b01261
7 Girometta C, Picco AM, Baiguera RM, et al. Physico-mechanical and thermodynamic properties of mycelium-based biocomposites: A review. Sustain. Published online 2019. doi:10.3390/su11010281
8 Kim SY, Go KC, Song YS, Jeong YS, Kim EJ, Kim BJ. Extract of the mycelium of T. matsutake inhibits elastase activity and TPA-induced MMP-1 expression in human fibroblasts. Int J Mol Med. Published online 2014. doi:10.3892/ijmm.2014.1969
9 Law JX, Liau LL, Saim A, Yang Y, Idrus R. Electrospun Collagen Nanofibers and Their Applications in Skin Tissue Engineering. Tissue Eng Regen Med. Published online 2017. doi:10.1007/s13770-017-0075-9
10 MacQueen LA, Alver CG, Chantre CO, et al. Muscle tissue engineering in fibrous gelatin: implications for meat analogs. npj Sci Food. Published online 2019. doi:10.1038/s41538-019- 0054-8
11 Enrione J, Blaker JJ, Brown DI, et al. Edible scaffolds based on non-mammalian biopolymers for myoblast growth. Materials (Basel). Published online 2017. doi:10.3390/ma10121404 12 Las Heras K, Santos-Vizcaino E, Garrido T, et al. Soy protein and chitin sponge-like scaffolds: From natural by-products to cell delivery systems for biomedical applications. Green Chem. Published online 2020. doi:10.1039/d0gc00089b
13 Ben-Arye T, Shandalov Y, Ben-Shaul S, et al. Textured soy protein scaffolds enable the generation of three-dimensional bovine skeletal muscle tissue for cell-based meat. Nat Food. Published online 2020. doi:10.1038/s43016-020-0046-5
14 Contessi Negrini N, Toffoletto N, Farè S, Altomare L. Plant Tissues as 3D Natural Scaffolds for Adipose, Bone and Tendon Tissue Regeneration. Front Bioeng Biotechnol. Published online 2020. doi:10.3389/fbioe.2020.00723
15 Campuzano S, Mogilever NB, Pelling AE. Decellularized plant-based scaffolds for guided alignment of myoblast cells. bioRxiv. Published online 2020. doi:10.1101/2020.02.23.958686 16 Hickey RJ, Pelling AE. Cellulose biomaterials for tissue engineering. Front Bioeng Biotechnol. Published online 2019. doi:10.3389/fbioe.2019.00045
17 Islam MR, Tudryn G, Bucinell R, Schadler L, Picu RC. Morphology and mechanics of fungal mycelium. Sci Rep. Published online 2017. doi:10.1038/s41598-017-13295-2
18 Rogalski JJ, Bastiaansen CWM, Peijs T. PA6 nanofibre production: A comparison between rotary jet spinning and electrospinning. Fibers. Published online 2018. doi:10.3390/fib6020037
19 Heimbuck AM, Priddy-Arrington TR, Sawyer BJ, Caldorera-Moore ME. Effects of postprocessing methods on chitosan-genipin hydrogel properties. Mater Sci Eng C. Published online 2019. doi:10.1016/j.msec.2018.12.119
20 Gungor-Ozkerim PS, Inci I, Zhang YS, Khademhosseini A, Dokmeci MR. Bioinks for 3D bioprinting: An overview. Biomater Sci. Published online 2018. doi:10.1039/c7bm00765e
21 Harvest RightTM | Home Freeze Dryers | Freeze Dried Food Storage Home Freeze Dryers. Accessed March 15, 2021. https://harvestright.com/product/pharmaceutical-freezedryer/? attribute_pa_attribute-size-pharm=size-largeattribute_pa_attribute-pump=oil-pump
22 Cannafreeze Freeze Dryer | Freeze Dry Machine Models Pricing. Accessed March 15, 2021. https://www.cannafreeze.net/models-pricing
23 Forced Air Lab Ovens - Cacade TEK. Accessed March 15, 2021. https://www.cascadetek.com/forced-air-lab-ovens/
24 ROKIT Dr. INVIVO 4D review - professional 3D bioprinter. Accessed March 15, 2021. https://www.aniwaa.com/product/3d-printers/rokit-dr-invivo-4d/
25 Advanced Solutions BioAssemblyBot review - 3D bioprinter. Accessed March 15, 2021. https://www.aniwaa.com/product/3d-printers/advanced-solutions-bioassemblybot/
26 James BD, Huya-Kouadio JM, Houchins C, Desantis DA. Mass Production Cost Estimation of Direct H 2 PEM Fuel Cell Systems for Transportation Applications: 2018 Update.; 2018. Accessed March 15, 2021. www.sainc.com
27 Winiski J, Mueller P. Bioreactor Paradigm for the Production of Secondary extra-Particle Hyphal Matrices Patent Application. Published online April 2, 2020. Accessed March 2, 2021. https://patents.justia.com/patent/20200102530
28 Aleph Farms and The Technion Reveal World’s First Cultivated Ribeye Steak. Published February 9, 2021. Accessed March 2, 2021. https://www.prnewswire.com/il/newsreleases/ aleph-farms-and-the-technion-reveal-worlds-first-cultivated-ribeye-steak- 301224800.html
29 Aleph Farms unveils prototype of first commercial cultivated steak product. Published November 18, 2020. Accessed March 2, 2021. https://www.prnewswire.com/il/newsreleases/ aleph-farms-unveils-prototype-of-first-commercial-cultivated-steak-product- 301175821.html
30 Ben-arye T, Levenberg S. CULTURED MEAT COMPOSITIONS. Published online May 7, 2020. 31 LAVON N, TOUBIA D, BODANOVSKY A. PLURIPOTENT CELL AGGREGATES AND USE THEREOF. Published online November 19, 2020.
32 LAVON N, KOREN I. HIGH QUALITY CULTURED MEAT, COMPOSITIONS AND METHODS FOR PRODUCING SAME. Published online May 22, 2020.
33 BlueNalu - Cell Based Tech. Accessed March 2, 2021. https://cellbasedtech.com/company/bluenalu
34 SAVIR I, FRIEDMAN S, BARAK K. CULTURED MEAT-CONTAINING HYBRID FOOD FIELD AND BACKGROUND OF THE INVENTION. Published online October 18, 2018.
35 BlueNalu raises $60m, gears up for commercial launch of cell-based seafood this year. Accessed March 15, 2021. https://www.foodnavigator-usa.com/Article/2021/01/20/BlueNaluraises- 60m-gears-up-for-commercial-launch-of-cell-based-seafood-this-year
36 Excell-ing in the lab — Ecovative Design. Accessed March 15, 2021. https://ecovativedesign.com/news/excell-ing-in-the-lab
37 Matrix Meats, Maker of Alt-Meat Scaffolding Tech, Raises Seed Funding. Accessed March 15, 2021. https://thespoon.tech/matrix-meats-maker-of-alt-meat-scaffolding-tech-raises-seedfunding/
38 Meati Foods raises $28m to expand its fungi-based protein platform. Accessed March 15, 2021. https://www.foodnavigator-usa.com/Article/2020/10/30/Meati-Foods-raises-28m-to-expandits-fungi-based-protein-platform
39 Wilgus TA. Growth Factor–Extracellular Matrix Interactions Regulate Wound Repair. Adv Wound Care. 2012;1(6):249-254. doi:10.1089/wound.2011.0344
40 Shahin-Shamsabadi A, Slvaganapathy PR. -SACS: PH Induced Self-Assembled Cell Sheets without the Need for Modified Surfaces. ACS Biomater Sci Eng. 2020;6(9):5346-5356. doi:10.1021/acsbiomaterials.0c01073
41 Velasco Barraza RD, Álvarez Suarez AS, Villarreal Gómez LJ, et al. REVISTA MEXICANA DE INGENIERÍA BIOMÉDICA ib Designing a Low Cost Electrospinning Device for Practical Learning in a Bioengineering Biomaterials Course. 2016;37(1):7-16. doi:10.17488/RMIB.37.1.1